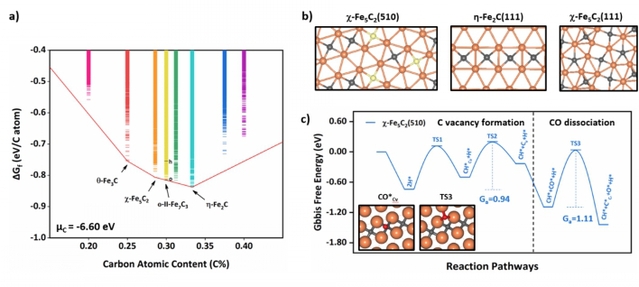
In situ-formed iron carbides (FeCx) are the key components responsible for Fischer–Tropsch synthesis (FTS, CO + H2→ long-chain hydrocarbons) on Fe-based catalysts in industry. The true active site is, however, highly controversial despite more than a century of study, which is largely due to the combined complexity in both FeCx structures and mechanism of CO hydrogenation. Herein powered by machine learning simulation, millions of structure candidates for FeCx bulk and surfaces are explored under FTS conditions, which leads to resolving the active site for CO activation. This is achieved without a priori input from experiment by first constructing the thermodynamics convex hull of bulk phases, followed by identifying the low surface energy surfaces and evaluating the adsorption ability of CO and H, and finally determining the lowest energy reaction pathway of CO activation. Rich information on FeCx structures and CO hydrogenation pathways is gleaned: (i) Fe5C2, Fe7C3, and Fe2C are the three stable bulk phases under FTS in producing olefins, where Fe7C3 and Fe2C have multiple energetically nearly degenerate bulk crystal phases; (ii) only three low surface energy surfaces of these bulk phases, namely, χ-Fe5C2(510), χ-Fe5C2(111), and η-Fe2C(111), expose the Fe sites that can adsorb H atoms exothermically, where the surface Fe:C ratio is 2, 1.75, and 2, respectively; (iii) CO activation via direct dissociation can occur at the surface C vacancies (e.g., with a barrier of 1.1 eV) that are created dynamically via hydrogenation. These atomic-level understandings facilitate the building of the structure–activity correlation and designing better FT catalysts.
https://pubs.acs.org/doi/10.1021/jacs.1c04624
