In recent years, the controlled assembly/disassembly of exogenous chemical components inside cells has become an emerging approach to regulating cell functions. However, the construction of dynamic material chemistry systems in living cells always remains highly challenging due to the complicated intracellular microenvironment. Nucleic acid is a category of biological components that can achieve efficient molecular assembly via specific base-pairing and perform biological functions in the intracellular microenvironment. Deoxyribonucleic acid (DNA) molecules exhibit the superior performance of intracellular assembly, including sequence programmability, molecule recognition ability, and nanostructure predictability, as well as the unique biological functions that traditional synthetic polymers do not carry, showing great superiority in the construction of dynamic material chemistry systems. Moreover, the technologies of DNA synthesis are relatively mature, and the conjugation of DNA with functional small molecules can be achieved through established chemical synthesis methods, facilitating the construction of DNA-based dynamic materials with more functions. In addition, a few specific DNA molecules have been proven to show responsiveness toward different stimuli, functioning as dynamic modules.
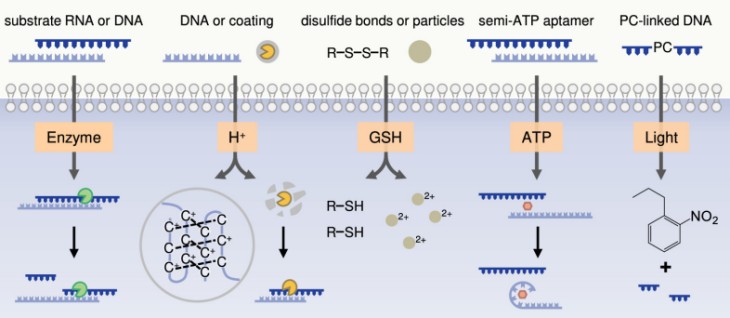
In this Account, we summarize our recent work in dynamic chemistry of DNA-based nanoassemblies in living cells from the perspective of stimulus types including enzyme, H+, glutathione (GSH), adenosine triphosphate (ATP), and light. Upon the specific stimuli, DNA-based nanoassemblies undergo precise assembly in living cells, executing disassembly or aggregation, which consequently affects the functions and behaviors of living cells. In the first part, we describe the interactions between DNA-based nanoassemblies and intracellular enzymes, namely the enzymatic cleavage of intracellular enzymes on the DNA or RNA sequences. In the second part, we summarize the effects of H+ in lysosomes on DNA-based nanoassemblies, including the formation of a tetraplex i-motif structure and the decomposition of acid-degradable polymeric coating. In the third part, we discuss the mechanism of GSH responsiveness of DNA-based nanoassemblies, including the breaking of disulfide bonds and reduction-responsive nanoparticles. In the fourth part, we describe the ATP-mediated conformational transition for the specific release of functional RNA sequences. In the fifth part, we demonstrate the light-mediated spatiotemporally dynamic chemistry of DNA-based nanoassemblies. In summary, based on the achievements of our group in the study of dynamic chemistry of DNA-based nanoassemblies, the assembly, disassembly, and reassembly in living cells are well-controlled, the regulation of cellular functions are explored, and the new strategies for cancer therapeutics are demonstrated. We envision that our work on the dynamic chemistry of DNA-based nanoassembly is a new paradigm for constructing dynamic material chemistry systems inside living cells, and will facilitate the development of precision medicine.
https://doi.org/10.1021/acs.accounts.4c00301