Focus News
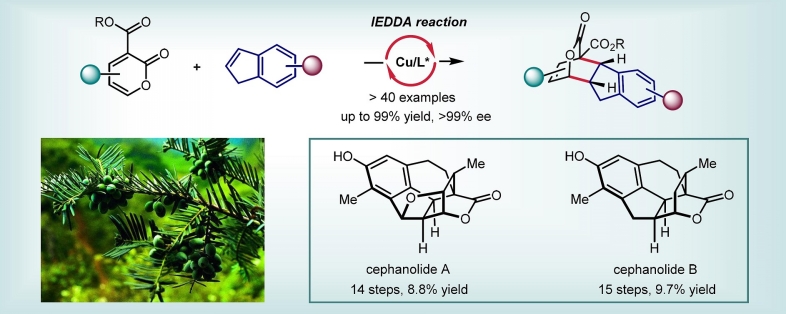
Catalytic Asymmetric Inverse-Electron-Demand Diels–Alder Reactions of 2-Pyrones with Indenes: Total Syntheses of Cephanolides A and B
Time:2021-12-13
An inverse-electron-demand Diels–Alder (IEDDA) reaction could complement the conventional normal-electron-demand Diels–Alder reaction in the synthesis of six-membered carbocycles. However, catalytic asymmetric all-carbon-based IEDDA reactions are underdeveloped. Herein, we disclosed a copper-catalyzed asymmetric IEDDA reaction using electron-deficient 3-carboalkoxyl-2-pyrones and electronically unbiased indenes as reactants. This method enables the rapid and enantioselective construction of a wide range of hexahydrofluorenyl bridged-lactone scaffolds. Using this method, asymmetric total syntheses of cephanolides A and B were accomplished.
https://onlinelibrary.wiley.com/doi/10.1002/anie.202112223