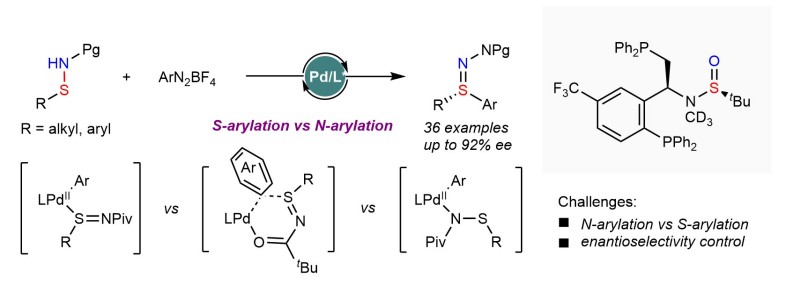
Sulfur-containing functional groups have garnered considerable attention owing to their frequent occurrence in ligands, pharmaceuticals and insecticides. However, enantioselective synthesis of sulfilimines, particularly diaryl sulfilimines remains a challenging and long-standing goal. Herein we report a highly enantio- and chemoselective cross-coupling of sulfenamides with aryl electrophiles to construct diverse S(IV) stereocenters by Pd catalysis. Bisphosphine ligands bearing sulfinamide groups were crucial for attaining high reactivity and selectivity. This strategy offers a general, modular and divergent platform for rapid synthesis of chiral sulfilimines and sulfoximines that are otherwise difficult to access. Furthermore, the origins of the high chemoselectivity and enantioselectivity were extensively investigated using density functional theory calculations.
https://doi.org/10.1002/anie.202409541