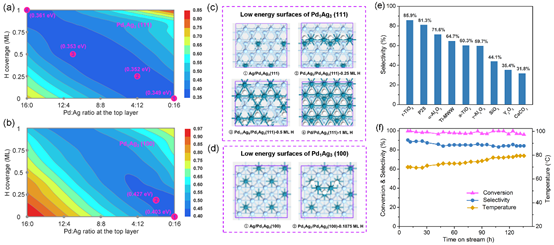
PdAg alloy is an industrial catalyst for acetylene-selective hydrogenation in excess ethene. While significant efforts have been devoted to increase the selectivity, there has been little progress in the catalyst performance at low temperatures. Here by combining a machine-learning atomic simulation and catalysis experiment, we clarify the surface status of PdAg alloy catalyst under the reaction conditions and screen out a rutile-TiO2 supported Pd1Ag3 catalyst with high performance: i.e., 85% selectivity at >96% acetylene conversion over a 100 h period in an experiment. The machine-learning global potential energy surface exploration determines the Pd-Ag-H bulk and surface phase diagrams under the reaction conditions, which reveals two key bulk compositions, Pd1Ag1 (R3̅m) and Pd1Ag3 (Pm3̅m), and quantifies the surface structures with varied Pd:Ag ratios under the reaction conditions. We show that the catalyst activity is controlled by the PdAg patterns on the (111) surface that are variable under reaction conditions, but the selectivity is largely determined by the amount of Pd exposure on the (100) surface. These insights provide the fundamental basis for the rational design of a better catalyst via three measures: (i) controlling the Pd:Ag ratio at 1:3, (ii) reducing the nanoparticle size to limit PdAg local patterns, (iii) searching for active supports to terminate the (100) facets.
https://pubs.acs.org/doi/10.1021/jacs.1c02471
