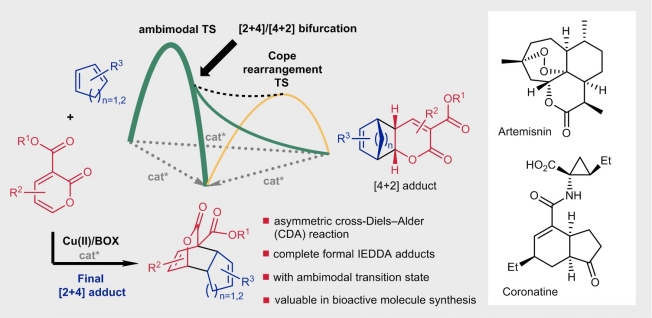
Compared with the conventional Diels–Alder reaction, the development of selective cross-Diels–Alder reactions between two different conjugated dienes, especially in a catalytic asymmetric manner, has been neglected. We now report a peri- and enantioselective cross-Diels–Alder reaction of 3-alkoxycarbonyl-2-pyrones with unactivated conjugated dienes catalysed by a copper(II)–bis(oxazoline) complex, leading to formal inverse-electron-demand adducts with high enantioselectivity under mild reaction conditions. Computational studies showed that this reaction proceeds through an ambimodal transition state: post-transition-state bifurcation leads to [2+4] and [4+2] adducts with the same enantioselectivity, followed by a facile Cope rearrangement to provide a single observed thermodynamic [2+4] product. This reaction occurs with a wide variety of cyclopentadienes, fulvenes and cyclohexadienes, providing a highly efficient and enantioselective approach to densely functionalized cis-bicyclic scaffolds. The synthetic value of this reaction is demonstrated by the asymmetric synthesis of two biologically important natural products, artemisinic acid and coronafacic acid.