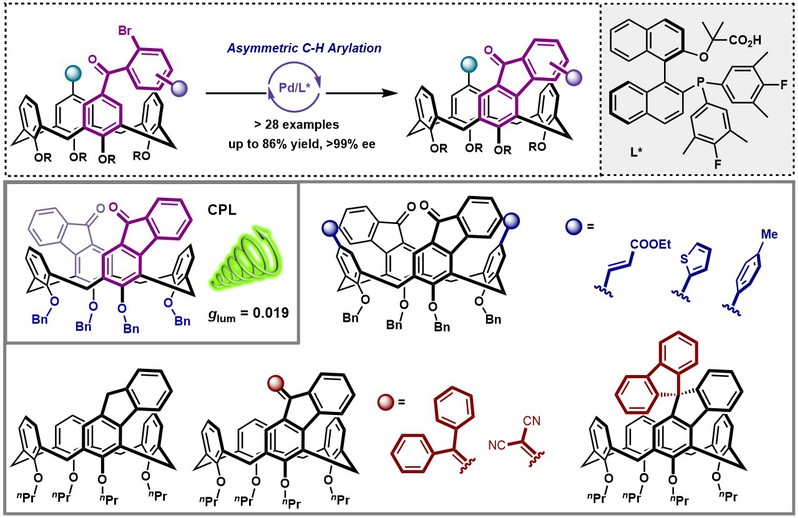
We report herein an efficient approach for the enantioselective synthesis of inherently chiral calix[4]arenes via palladium-catalyzed asymmetric intramolecular C–H arylations. Using a chiral bifunctional phosphine-carboxylate ligand, the inherent chirality on macrocyclic scaffolds was induced successfully, from which a wide range of calix[4]arenes with fluorenone motifs were obtained with good yields and excellent enantioselectivities (up to >99% ee). The synthetic utility of this method was demonstrated by diverse transformations of the products, thus substantially expanding the chemical space of chiral calix[4]arenes. Further investigations of photophysical and chiroptical properties revealed that calix[4]arenes bearing two fluorenone moieties displayed remarkable glum values (up to 0.019), highlighting the great potential of inherent chirality in the development of organic optoelectronic materials.
https://pubs.acs.org/doi/10.1021/jacs.2c10606