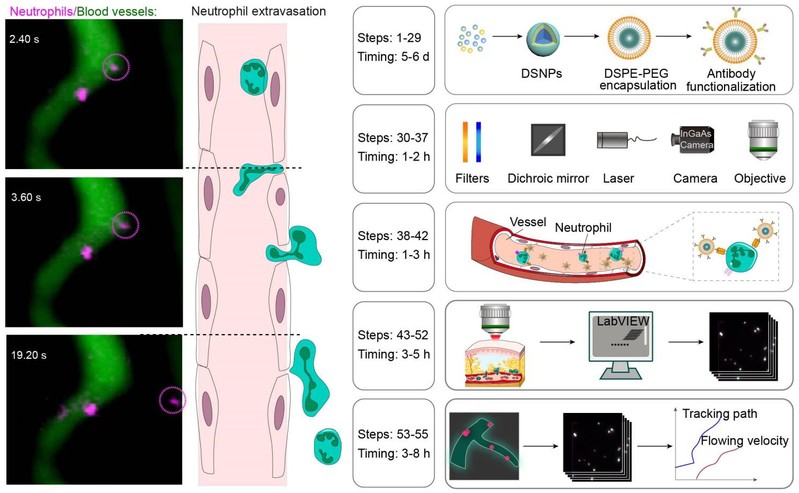
In vivo microscopy of single cells enables following pathological changes in tissues, revealing signaling networks and cell interactions critical to disease progression. However, conventional intravital microscopy at visible and near-infrared wavelengths <900 nm (NIR-I) suffers from attenuation and is typically performed following the surgical creation of an imaging window. Such surgical procedures cause the alteration of the local vasculature and induce inflammation in skin, muscle and skull, inevitably altering the microenvironment in the imaging area. Here, we detail the use of near-infrared fluorescence (NIR-II, 1,000–1,700 nm) for in vivo microscopy to circumvent attenuation in living tissues. This approach enables the noninvasive visualization of cell migration in deep tissues by labeling specific cells with NIR-II lanthanide downshifting nanoparticles exhibiting high physicochemical stability and photostability. We further developed a NIR-II fluorescence microscopy setup for in vivo imaging through the intact skull with high spatiotemporal resolution, which we use for the real-time dynamic visualization of single-neutrophil behavior in the deep brain of a mouse model of ischemic stroke. The labeled downshifting nanoparticle synthesis takes 5–6 d, the imaging system setup takes 1–2 h, the in vivo cell labeling takes 1–3 h, the in vivo NIR-II microscopic imaging takes 3–5 h and the data analysis takes 3–8 h. The procedures can be performed by users with standard laboratory training in nanomaterials research and appropriate animal handling.
https://www.nature.com/articles/s41596-024-00983-3